Reduced intensity of many GI Adverse Events
Switching to once-weekly subcutaneous methotrexate reduced gastrointestinal (GI) adverse events (AEs) associated with oral methotrexate1
In a survey, adult RA patients (N = 70) with GI side effects from oral methotrexate experienced a reduction in the intensity of most measured GI AEs after switching to subcutaneous methotrexate.1 Study was not conducted with Otrexup.
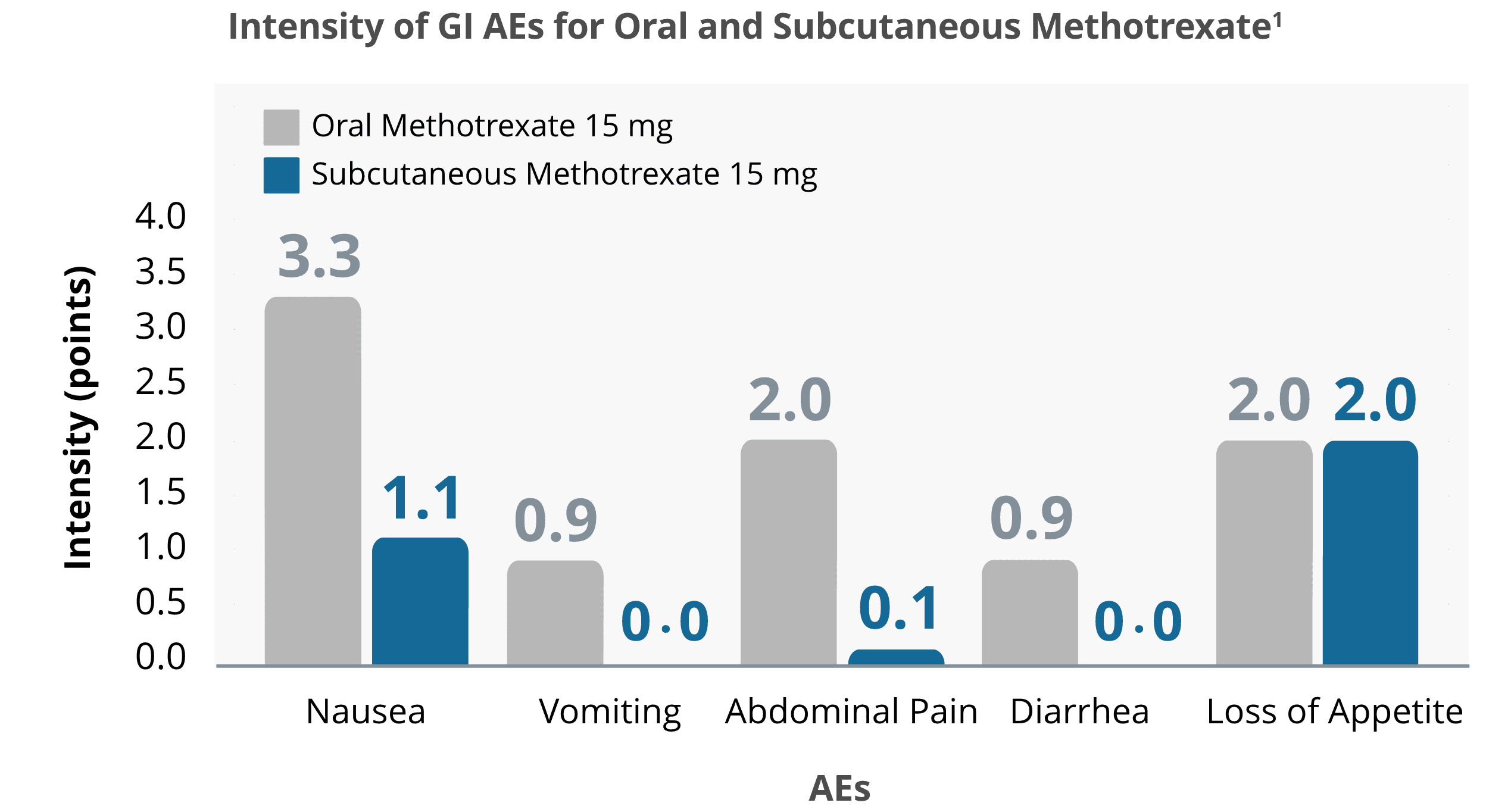
Source: Adapted from Rutkowska-Sak, et al. 2009.
Otrexup was approved through the 505(b)(2) pathway, which means it demonstrated bioequivalence to a subcutaneous methotrexate (10 mg, 15 mg, 20 mg, and 25 mg) previously approved by the FDA. Safety and efficacy clinical trials were not conducted with Otrexup.
Subcutaneous methotrexate delivered higher bioavailability than oral methotrexate2,3
Bioavailability following oral dosing showed a plateau effect at doses of 15 mg and greater:
In adults, oral absorption appears to be dose dependent. Peak serum levels are reached within 1 to 2 hours. At doses of 30 mg/m2 or less, methotrexate is generally well absorbed with a mean bioavailability of about 60%. The absorption of doses greater than 80 mg/m2 is significantly less, possibly due to a saturation effect.
In relative bioavailability studies in RA patients, systemic exposure of methotrexate was found to be similar between Otrexup and intramuscular or subcutaneous administration of methotrexate injection at the same doses; however, systemic exposure of methotrexate was higher with Otrexup as compared to oral administration of methotrexate at the same dose.
Bioavailability following oral dosing showed a plateau effect at doses of 15 mg and greater. The systemic exposure of methotrexate from Otrexup at doses of 10, 15, 20, and 25 mg was higher than that of oral methotrexate by 17, 13, 31, and 36%, respectively. Methotrexate systemic absorption from Otrexup was similar when administered into the abdomen or thigh.
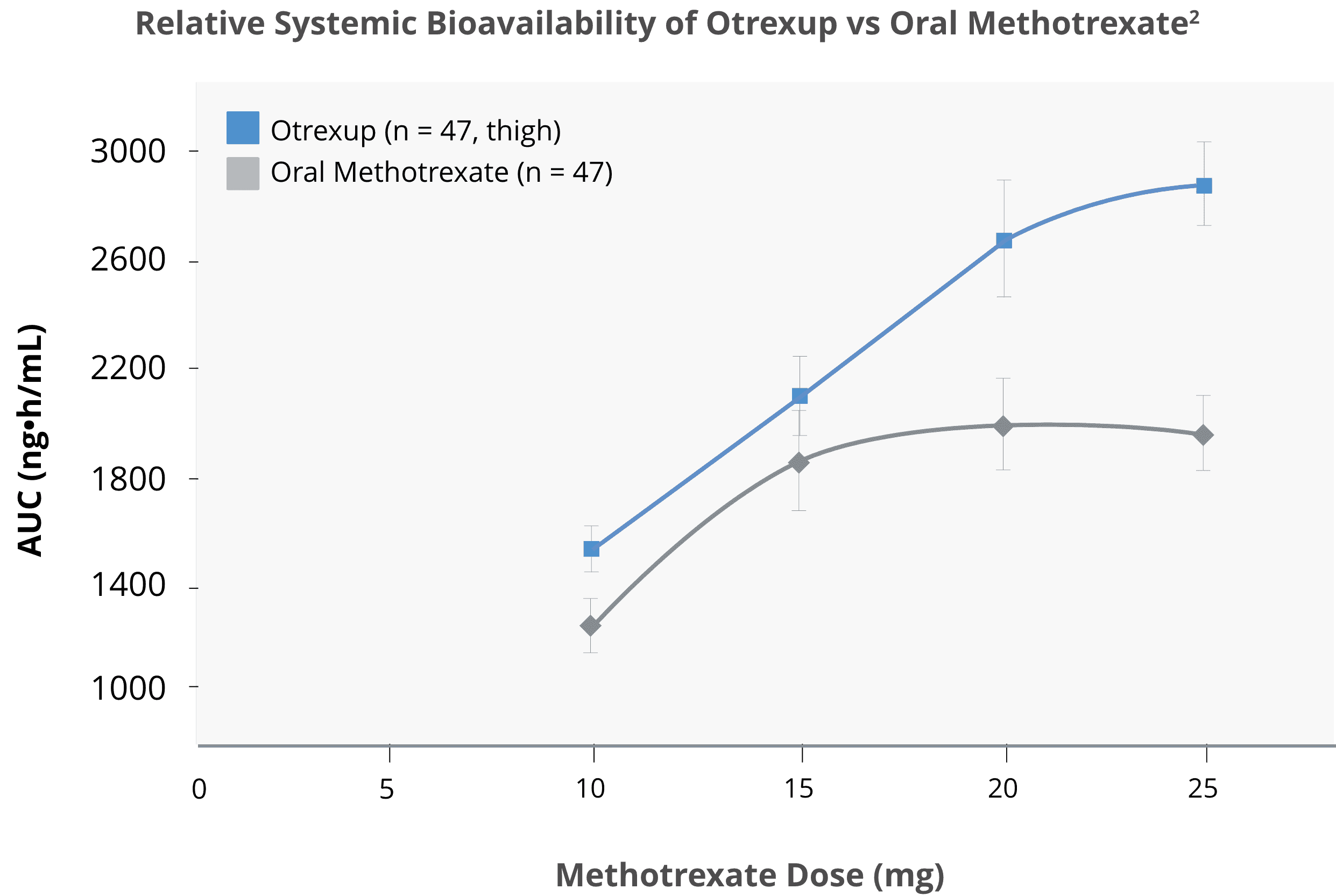
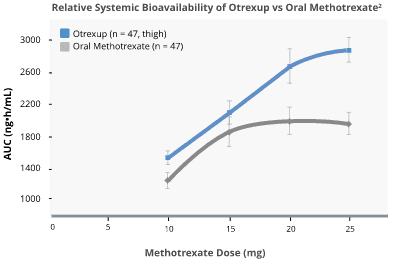
Source: Adapted from Schiff, et al. 2014.
Otrexup autoinjection can be an easy-to-use option

In a study of 101 adult RA patients, 98% agreed that Otrexup was easy to use.4
Phase 2 clinical trial in RA patients with moderate to severe functional limitations.*4

Patients reported no or minimal administration-site pain during the study, as reflected by visual analog scale (VAS) pain scores.4
Out of total adult RA patients (N = 101) treated, 55 and 74 patients reported no or minimal administration-site pain on Day 1 and Day 2, respectively (VAS 0 mm = no pain, 100 mm = worst possible pain). Mean pain score on Day 1 was 3.6 mm and 1.4 mm on Day 2 (using VAS 0 mm to 100 mm) in adult RA patients.*4
Otrexup is designed so the needle is not visible to the patient.4

With Otrexup, less of the bright yellow methotrexate is visible.
Small medication window, minimal view of yellow methotrexate.
*A phase 2, multicenter, open-label, single-dose, single-arm study in adult RA patients (N = 101) with moderate to severe functional limitations received standardized subcutaneous (SC) self-injection training. Most patients (80.2%) had previous experience with SC administration; 31 had used an autoinjector, 24 had used a pen device, and 53 had used a needle and syringe with a vial. Approximately 77% were receiving oral tablets of MTX at enrollment.4
Meet the Otrexup autoinjector


AE, adverse event; GI, gastrointestinal; pJIA, polyarticular juvenile idiopathic arthritis; RA, rheumatoid arthritis; SC, subcutaneous.
References:
1. Rutkowska-Sak L, Rell-Bakalarska M, Lisowska B. Oral vs. subcutaneous low-dose methotrexate treatment in reducing gastrointestinal side effects. Reumatologia. 2009;47(4):207-211. 2. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73(8):1549-1551. 3. Otrexup. Prescribing information. Antares Pharma Inc; 2019. 4. Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20(5):256-260.
