What is OTREXUP? (Indications)
OTREXUP is a single-dose auto-injector containing a prescription medicine, methotrexate.1
Methotrexate is used to1:
- Treat certain adults with severe, active rheumatoid arthritis (RA), and children with active polyarticular juvenile idiopathic arthritis (pJIA), after treatment with other medicines including non-steroidal anti-inflammatory drugs (NSAIDs) have been used and did not work well.
- Control the symptoms of severe, resistant, disabling psoriasis in adults when other types of treatment have been used and did not work well.
OTREXUP should not be used for the treatment of cancer.1
OTREXUP should not be used for the treatment of children with psoriasis.1
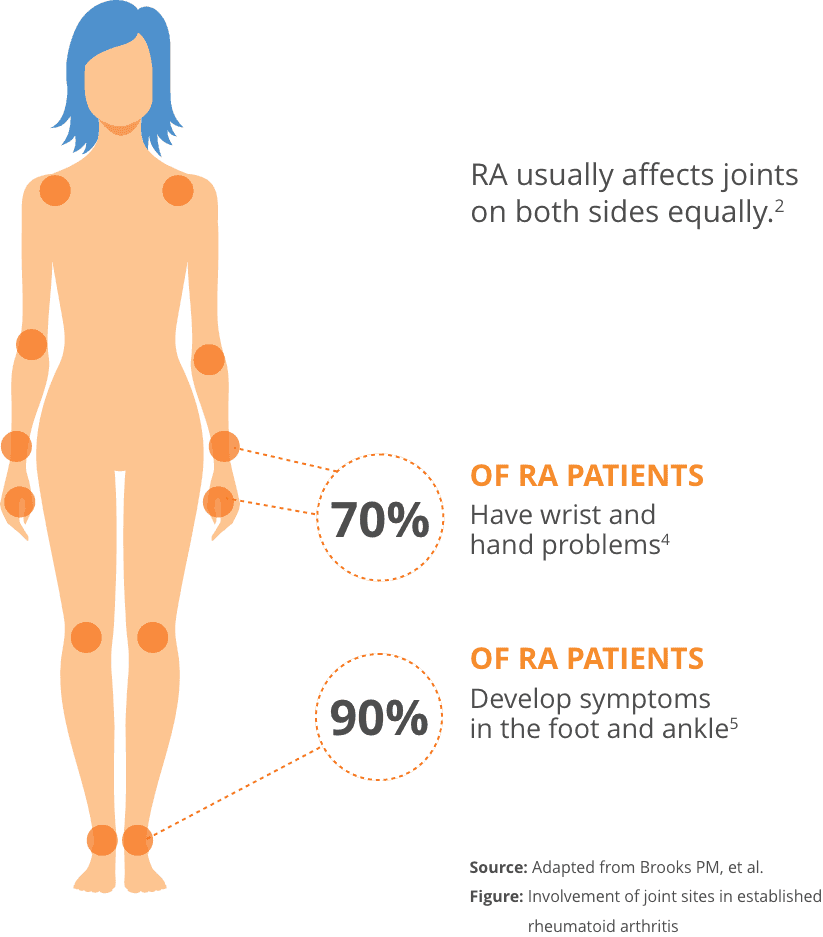
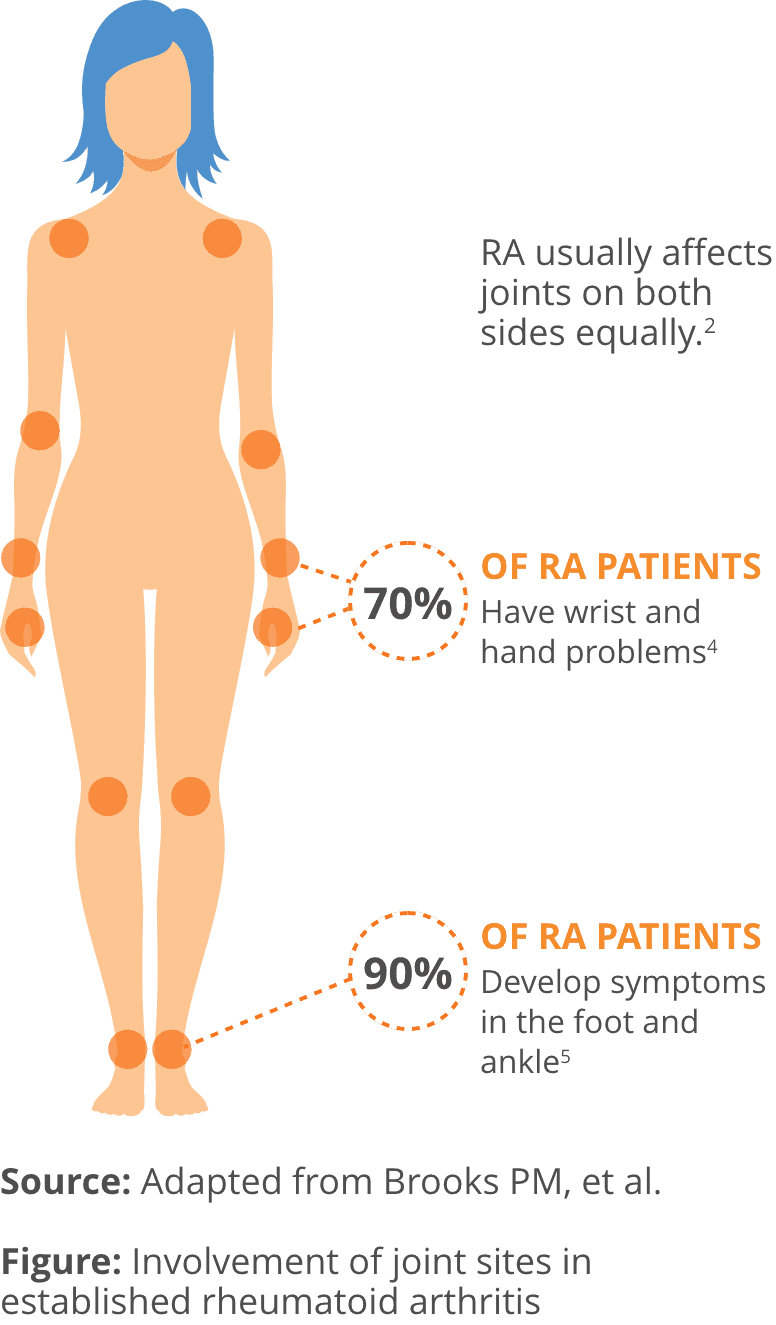
- RA is a type of autoimmune disease, where the body’s own immune system mistakenly attacks healthy cells instead of unhealthy one2
- RA affects approximately 1.3 million Americans. About 75% of RA patients are women2,3
- RA generally begins between the ages of 30 and 50. However, RA can start at any age2
- RA causes pain, swelling, and reduced range of motion in the wrist and small joints of the hands and feet, but it can also affect the shoulders and knees2
- Juvenile idiopathic arthritis (JIA) is the most common arthritis in children under the age of 16. It affects almost 1 in 1000 children in the US6
- JIA can cause joint pain, swelling, and stiffness. Some children have symptoms for only a few months, whereas others can have symptoms for years7
- Most children with JIA have the polyarticular form (pJIA), which means having arthritis in 5 or more joints8
- Psoriasis is a chronic skin disease9
- Psoriasis can cause rashes with itchy, scaly patches, most commonly on the knees, elbows, trunk, and scalp9
- According to the National Psoriasis Foundation, approximately 7.5 million Americans have psoriasis10
Meet the Otrexup autoinjector


Otrexup autoinjection can be an easy-to-use option

In a study of 101 adult RA patients, 98% agreed that Otrexup was easy to use.11
In adult RA patients with moderate to severe functional limitations, most of them (80.2%) had previous experience with SC (under the skin) administration; 31 had used an autoinjector, 24 had used a pen device, and 53 had used a needle and syringe with a vial. Approximately 77% were receiving oral tablets of methotrexate at enrollment.11

Patients experienced no or minimal pain.11
In a clinical study of 101 adult RA patients, 55 and 74 patients reported no or minimal administration-site pain on Day 1 and Day 2, respectively.11
Otrexup is designed so the needle is not visible.11

With Otrexup, less of the bright yellow methotrexate is visible.
Small medication window, minimal view of yellow methotrexate.
Otrexup general steps for administration1
Before giving the injection, see patient instructions for use for complete administration instructions.
Step 1
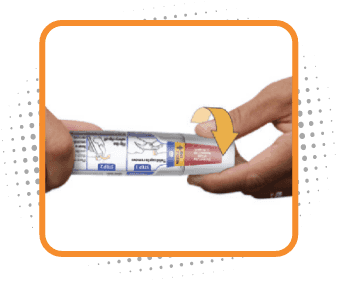
Remove cap
- Twist cap marked 1 to remove. This will break the seal
- Do not replace the cap after it has been removed
- After the cap is removed, Otrexup must be used or disposed of safely
Step 2
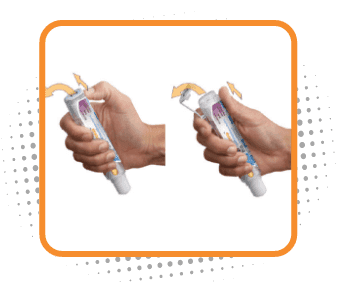
Remove the safety clip
- Flip the safety clip marked 2
Step 3
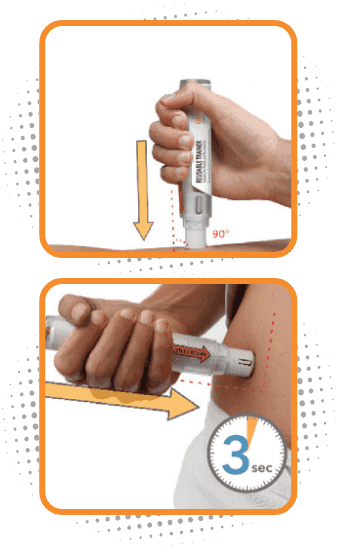
Administer Otrexup in the thigh or abdomen
- Place the needle end of Otrexup flat against thigh or stomach at a 90° and firmly push device against the injection site until fully depressed. You will hear a click, then hold for 3 seconds
- Do not inject Otrexup within 2 inches of the belly button (navel)
- Do not inject Otrexup in the arms or any other areas of the body
- You may notice a small amount of blood or liquid at the administration site, which is normal
- Remove Otrexup from the site and press a cotton ball on the area for 10 seconds. Do not rub the area
Going in for a checkup? Start a conversation with your doctor about Otrexup!
JIA, juvenile idiopathic arthritis; NSAIDs, non-steroidal anti-inflammatory drugs; pJIA, polyarticular juvenile idiopathic arthritis; RA, rheumatoid arthritis; SC, subcutaneous.
References:
1. Otrexup. Prescribing information. Antares Pharma Inc; 2019. 2. Rheumatoid Arthritis. American College of Rheumatology. Accessed September 26, 2022. https://rheumatology.org/patients/rheumatoid-arthritis. 3. Xu Y, Wu Q. Prevalence Trend and Disparities in Rheumatoid Arthritis among US Adults, 2005-2018. J Clin Med. 2021;10(15):3289. 4. Chung KC, Pushman AG. Current concepts in the management of the rheumatoid hand. J Hand Surg Am. 2011;36(4):736-747. 5. American Academy of Orthopedic Surgeons. Rheumatoid Arthritis of Foot and Ankle. OrthoInfo. Accessed September 26, 2022. https://orthoinfo.aaos.org/en/diseases--conditions/rheumatoid-arthritis-of-the-foot-and-ankle. 6. Wallace CA, Giannini EH, Spalding SJ, et al. Childhood Arthritis and Rheumatology Research Alliance. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64(6):2012-2021. 7. Juvenile idiopathic arthritis. Mayo Clinic. Accessed September 26, 2022. https://www.mayoclinic.org/diseases-conditions/juvenile-idiopathic-arthritis/symptoms-causes/syc-20374082. 8. Ringold S, Weiss PF, Colbert RA, et al. Juvenile Idiopathic Arthritis Research Committee of the Childhood Arthritis and Rheumatology Research Alliance. Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2014;66(7):1063-1072. 9. Psoriasis. Mayo Clinic. Accessed September 26, 2022. https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840. 10. Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis Prevalence in Adults in the United States. JAMA Dermatol. 2021;157(8):940-946. 11. Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20(5):256-260.
